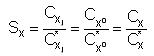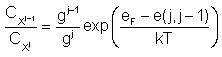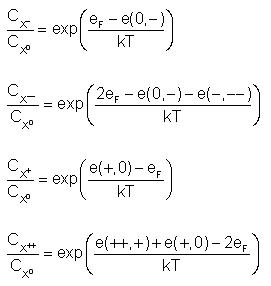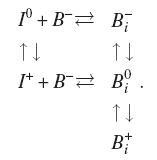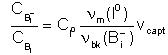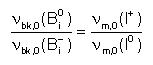Concentrations
 Equilibrium
concentration of neutral native defects
Equilibrium
concentration of neutral native defects
The
concentration of
single neutral native
defects under equilibrium conditions is determined
by:
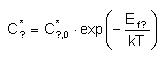
Where:
- C?,0* is the prefactor, related to DADOS parameter
C0relEq_? under equilibrium
conditions as follows:
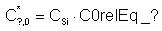
- Ef? is the formation energy, that is equal to the input
parameter
Eform_?.
- See symbol list.
 Supersaturation
Supersaturation
Supersaturation is defined for both interstitials and vacancies as the ration
between its concentration and the equilibrium one.
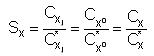
Supersaturation does not depend on the charge state. This is because the
rations Cj-1/Cj do not depend on the excess of defects,
but only on Fermi level (Fahey
et al. 1989).
 Concentration ratio of charged
species
Concentration ratio of charged
species
The relative concentration between the of
charged species is:
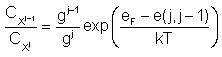
Where:
- CXj is the concentration of X
with charge "j".
- gj is the degeneracy factor. We assume it to be the same for
all charge states, i.e. there is no entropy change associated to charge
transitions.
- eF is the Fermi level.
- e(j,j-1) is the electronic level for species X at temperature T. In the
DDP file, this
parameter is
defined but for T = 0 K.
- See symbol list.
According to the equation, the following expressions are valid:
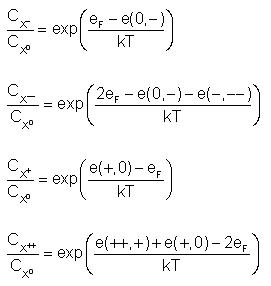
See Fahey et al. 1989 and
Martin-Bragado et al. 2005.
 Break-up of charged pairs: Boron
case
Break-up of charged pairs: Boron
case
The pairing, break-up and charge reactions related to Bi are
represented by the reactions:
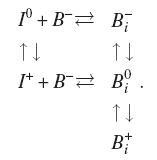
Comments
- Substitutional boron is always immobile and ionized (B-).
- Horizontal reactions (pairing and break-up) conserve the charge while
vertical reactions establish the electrical equilibrium.
- The rate of the horizontal reactions will not depend on
Fermi level.
- Direct break-up of Bi+ is not included because I++
is not implemented.
- The activation energy for Bi- break-up will be Eb(Bi-)+Em(I0)
(see figure below), where Eb is the binding energy and
Em is the migration
energy.
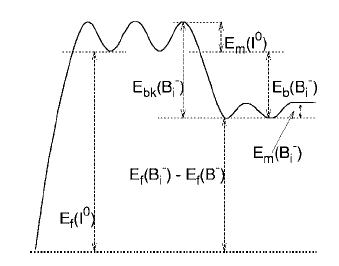
- Considering energy conservation, we can conclude: Eb(Bi0)=Eb(Bi-)+eBi(0,-)-eI(+,0),where
e(j,j-1) is the electronic level for species at temperature T. Electronic levels
scale with gap energy, introducing a slight temperature dependance in binding
energy for Bi0.
- The number of broken Bi- per unit of volume and time
will be CBi-nbk(Bi-)
and the number of new formed CB-CI0νm(I0)vcapt,
where vcapt is the effective capture volume for pairing reaction.
Consequently, in local equilibrium conditions:
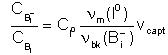
- And, from equilibrium conditions it can be also derived that the break-up
prefactors, νbk,0 must fulfill:
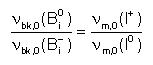
See Martin-Bragado et al. 2005.
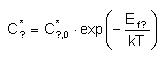
![]()